Abstract
Background: Since the 1960s, an increased risk of SPMs in multiple myeloma (MM) patients has been noted but less consequential due to relatively short survival. Given considerably improved survival as well as the potential association with modern anti-myeloma therapy, reassessment of this risk is prudent. Prior studies of aHSCT recipients have calculated standardized incidence ratios compared to the general population, but have not compared the cumulative incidence of SPMs among myeloma patients undergoing aHSCT to those who do not. Thus, a direct comparison of the effect of aHSCT on development of SPMs in MM patients is necessary to better advise patients and physicians on the potential risks of this treatment in the modern era.
Methods: Patients diagnosed with MM between 1991 and 2013 were identified in the California Cancer Registry linked to the California hospital Patient Discharge Database. Inclusion criteria included a first primary diagnosis of MM and a minimum 1 year of follow up. SPMs were defined as distinct malignancies arising ≥1 year after MM diagnosis. The cumulative incidence (CI) of developing any SPM, solid tumors, and hematologic malignancies, and associated 95% confidence intervals were computed, accounting for the competing risk of death. To compare the cumulative incidence of SPMs between aHSCT recipients and those without aHSCT, patients were matched 1:2 on age, year of diagnosis, race/ethnicity, socioeconomic status and comorbidities. Multivariable cox proportional hazards regression models, using the methods of Fine and Gray to account for competing risk of death, and stratified for variables that did not meet the proportional hazards assumption, estimated the effect of aHSCT on the risk of SPM development accounting for baseline demographics.
Results: Among 16,331 patients, 933 (5.7%) developed a SPM ≥1 year after diagnosis. Of these, 119 (12.8%) were hematologic malignancies, primarily leukemia (81, 9%). The most common solid tumors were malignancies of the digestive (184, 19.7%), respiratory (119, 12.8%), and male genital (115, 12.3%) systems. An additional 310 SPMs were diagnosed <1 year after diagnosis and were excluded from further analysis. There was no difference in aHSCT utilization among those who developed SPMs (186, 19.9%) and those do did not develop a SPM (3016, 19.6%) (P=0.79).
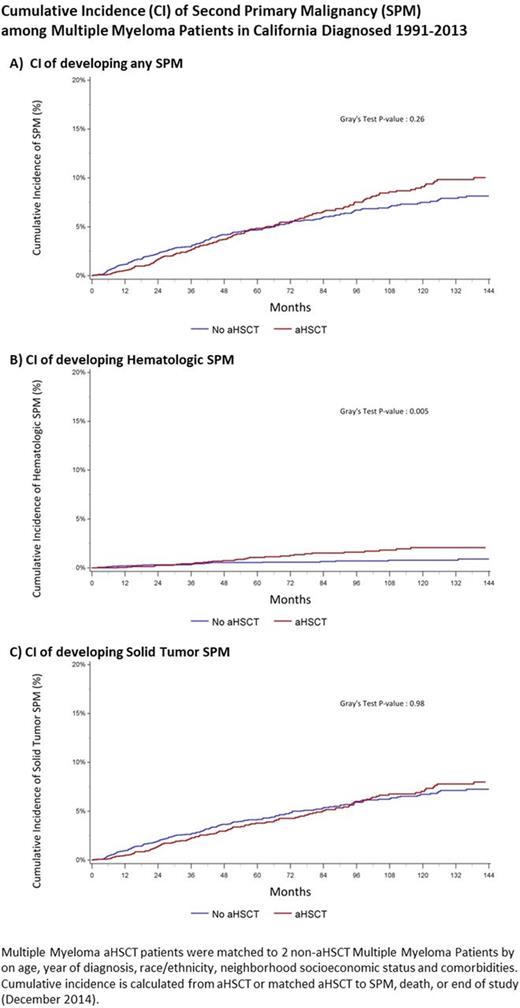
The 5- and 10-year CI of developing any SPM, accounting for the competing risk of death, was 4% (3.7% - 4.3%) and 6.6% (6.2% - 7.1%) respectively. There is no plateau in the incidence of SPM development (Figure). The 5- and 10-year cumulative incidence of developing solid SPM was 3.5% (3.3% - 3.9%) and 5.7% (5.3% - 6.2%). The 5- and 10-year cumulative incidence of developing hematologic SPM was 0.5% (0.4% - 0.6%) and 0.9% (0.7% - 1.1%).
In the matched analysis, the 5- and 10-year CI of developing a SPM was similar among aHSCT recipients [5-year: 4.8% (3.6% - 5.9%); 10-year: 9.1% (7.7% - 10.7%)] and among non-aHSCT recipients [5-year: 4.7% (4.0% - 5.5%); 10-year: 7.5% (6.5% - 8.6%)] (Figure Part A) (P=0.26). However, the CI of hematologic SPMs differed among the aHSCT and non-aHSCT groups reaching 2.1% (1.4% - 2.9%) among aHSCT recipients vs 0.8% (0.5% - 1.2%) at 10 years (P = 0.005). There was no difference in the CI of solid SPMs between the two groups (Figure C) (P=0.98).
Multivariable models adjusted for baseline characteristics and the competing of risk of death identified no increased risk of developing SPMs overall among aHSCT recipients (adjusted hazard ratio (aHR) 1.13, 95% CI 0.94 - 1.37). The risk of developing hematologic SPMs was increased in the aHSCT recipients (aHR 1.65, 1.03 - 2.63), while the risk of developing solid SPMs was not (aHR 1.06, 0.86 - 1.31). Era of diagnosis (1991-97, 1998-2002, 2003-07, 2008-13) was not associated with an increased risk of SPMs.
Conclusions: MM patients are at risk for SPMs and we observed that the cumulative incidence increases over time. The use of aHSCT as part of treatment and the era of diagnosis were not associated with a change in risk of overall SPM development. Though no difference in SPM development over time was noted, follow up time during eras when continuous therapy was more common are still short. A small but significant increase in hematologic SPMs was noted among aHSCT recipients. As MM patients continue to live longer, efforts to effectively screen and prevent SPMs are warranted.
Rosenberg: Amgen: Speakers Bureau. Tuscano: Novartis: Research Funding; Spectrum: Research Funding; Celgene: Research Funding, Speakers Bureau; Takeda: Research Funding; Amgen: Speakers Bureau. Jonas: AbbVie, Celgene, Daiichi Sankyo, Pharmacyclics, Genentech/Roche, Glycomimetics, Esanex, Kalobios: Research Funding; Rigel: Consultancy; Celgene: Membership on an entity's Board of Directors or advisory committees. Abedi: Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; California Institute of Regenerative Medicine: Research Funding; Seattle Genetics: Speakers Bureau; Amgen: Research Funding; BMS: Speakers Bureau; Takeda: Speakers Bureau; Gilead: Speakers Bureau. Wun: Pfizer: Other: Steering Committee: RESET study, Research Funding; Janssen: Other: Steering Committee for Cassini Study and Callisto Program, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal